boron number of protons electrons and neutrons|Boron : Cebu Discover the fascinating world of carbon, the king of the elements, and learn . Login ID Your login ID may vary depending upon your paystub system. Legacy Lifepoint team members should enter their Lifepoint 3-4 ID or UltiPro ID (with leading zeros). Legacy Kindred team members should enter their legacy Kindred domain ID.
PH0 · How to find the Number of Protons, Electrons, Neutrons for Boron
PH1 · How to Find the Number of Protons, Neutrons, and
PH2 · How many electrons does boron have?
PH3 · Chemical Elements.com
PH4 · Boron Protons Neutrons Electrons (And How to Find them?)
PH5 · Boron Protons Neutrons Electrons (And How to Find them?)
PH6 · Boron (B)
PH7 · Boron
The standout features of Essentials of Strategic Management, 7th edition, are its concisely written and robust coverage of strategic management concepts and compelling collection of cases. The text presents a conceptually strong treatment of strategic management principles
boron number of protons electrons and neutrons*******The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Isotopes Atoms of the same element .boron number of protons electrons and neutrons Boron Discover beryllium, a silvery-white metal with unique properties and applications. .Discover the fascinating world of carbon, the king of the elements, and learn .Boron – Protons – Neutrons – Electrons – Electron Configuration. Natural boron consists primarily of two stable isotopes, 11B (80.1%) .
In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Boron (B). From the Periodic Table .
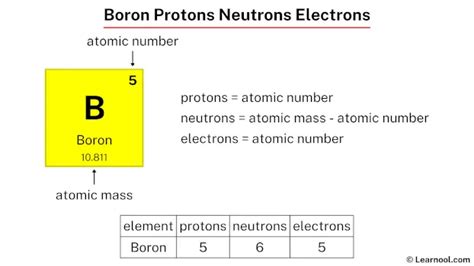
Boron is the 5th element in the periodic table and has a symbol of B and atomic number of 5. It has an atomic weight of 10.810 and a mass number of 11. Boron has five protons .Name: Boron. Symbol: B. Atomic Number: 5. Atomic Mass: 10.811 amu. Melting Point: 2300.0 °C (2573.15 K, 4172.0 °F) Boiling Point: 2550.0 °C (2823.15 K, 4622.0 °F) Number of Protons/Electrons: 5. Number of .Atomic structure. Boron is the lightest element having an electron in a p-orbital in its ground state. Unlike most other p-elements, it rarely obeys the octet rule and usually places only six electrons [44] (in three .
Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure. The chemical symbol for Boron is B. .
The atomic number (number at the top) is the amount of protons and the amount of electrons. So if an element has an atomic number of 5, you know that it has 5 protons and 5 electrons. The . Boron atomic number 5 has five electrons in its ground state. Commonly Boron will lose 3 electrons leaving 2 electrons in its most common ionic form. The atomic number gives the number of protons. . Boron has 5 protons, 6 neutrons and 5 electrons. But how will you find the number of protons, neutrons and electrons in Boron (B)? Well, it is very easy to find the .
Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure.The chemical symbol for Boron is B. Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. There are over 100 different borate minerals, but the most common are: borax, .
While protons and neutrons are located inside the nucleus at the center of the atom, electrons are located outside the nucleus in what is often called the electron cloud. Figure 4.4.1 4.4. 1: Electrons are much smaller than protons or neutrons. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling .
The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons. As will .
Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element.
Name: Boron Symbol: B Atomic Number: 5 Atomic Mass: 10.811 amu Melting Point: 2300.0 °C (2573.15 K, 4172.0 °F) Boiling Point: 2550.0 °C (2823.15 K, 4622.0 °F) Number of Protons/Electrons: 5 Number of Neutrons: 6 Classification: Metalloid Crystal Structure: Rhombohedral Density @ 293 K: 2.34 g/cm 3 Color: brownish Atomic Structureboron number of protons electrons and neutronsName of the isotope: Boron-10; B-10 Symbol: 10 B or 105 B Mass number A: 10 (= number of nucleons) Atomic number Z: 5 (= number of protons) Neutrons N: 5 Isotopic mass: 10.012937 (3) u ( atomic weight of Boron-10) Nuclide mass: 10.0101941 u (calculated nuclear mass without electrons) Mass excess: 12.05074 MeV Mass defect: .
Watch this video to learn how protons, neutrons, and electrons are arranged in atoms, and how the number and distribution of these subatomic particles determine the identity and properties of . The atomic number tells you how many protons an atom has. The top number is the mass number (10). The mass number tells you the mass of the atom. The mass of the atom is the combined number of protons and neutrons. Electrons don't contribute much mass at all, so we ignore them for questions like this. To find the . Mass Number = # of Protons + # of Neutrons. Mass Number = 1 + 2. Therefore, this particular atom of hydrogen will have a mass number of 3. Note that the mass number calculated in Example 2.4.1 2.4. 1 does not match the number underneath the elemental symbol and name for hydrogen on the periodic table.Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. The relative masses of atoms are reported using the atomic mass unit ( amu ), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons.
Boron Electrons always provide a negative charge. It is expressed by e –. The charge of electrons is –1.609 × 10 –19 coulombs and the relative charge is –1. That is, the charge of an electron is equal to that of a proton but the opposite. Try the Proton Neutron Electron Calculator and get instant results for any element.
That's where atomic number and mass number are useful. Figure 4.5.1 4.5. 1: It is difficult to find qualities that differ between each element, and to distinguish one element from another. Each element, however, does have a unique number of protons. Sulfur has 16 protons, silicon has 14 protons, and gold has 79 protons.
Calculate the number of protons, neutrons and electrons it contains. Show answer Hide answer. Number of protons = 11. Number of electrons = 11. Number of neutrons (mass number - atomic number .Figure 3.5.1 3.5. 1: Unlike protons, the number of neutrons is not absolutely fixed for most elements. Atoms that have the same number of protons, and hence the same atomic number, but different numbers of neutrons are called isotopes. All isotopes of an element have the same number of protons and electrons, which means they exhibit the same . This chemistry video tutorial explains how to calculate the number of protons, neutrons, and electrons in an atom or in an ion. It also explains the differe. Figure 3.4.1 3.4. 1: The social security number subatomic-the proton. Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons. Lithium has 3 protons, 4 neutrons and 3 electrons. 4. Beryllium has 4 protons, 5 neutrons and 4 electrons. 5. Boron has 5 protons, 6 neutrons and 5 electrons. 6.
The Shadowverse Club Tournament begins! Light Tenryu came to Shadowverse College without knowing the first thing about Shadowverse and joined Seventh Flame, a Shadowverse club on the verge of being shut down. He falls in love with playing Shadowverse while working with his friends Subaru Makabe and Itsuki .
boron number of protons electrons and neutrons|Boron